What is Diethyl Ether ?
A molecule that revolutionized medicine with its simplicity and volatility.
How a Simple Molecule Changed the Way We Feel Pain
The intersection of Chemistry with Physiology

A molecule that revolutionized medicine with its simplicity and volatility.

The intersection of Chemistry with Physiology

Diethyl ether is a volatile, flammable liquid with a low boiling point of around 35 degrees Celsius. Its lipid solubility allows it to rapidly cross the blood brain barrier, making it an effective general anaesthetic. Once inhaled, ether acts on neuronal membranes and ion channels, suppressing brain activity and rendering the patient unconscious. Though once used recreationally, its medical significance emerged in the nineteenth century, as it enabled painless surgical procedures for the first time.
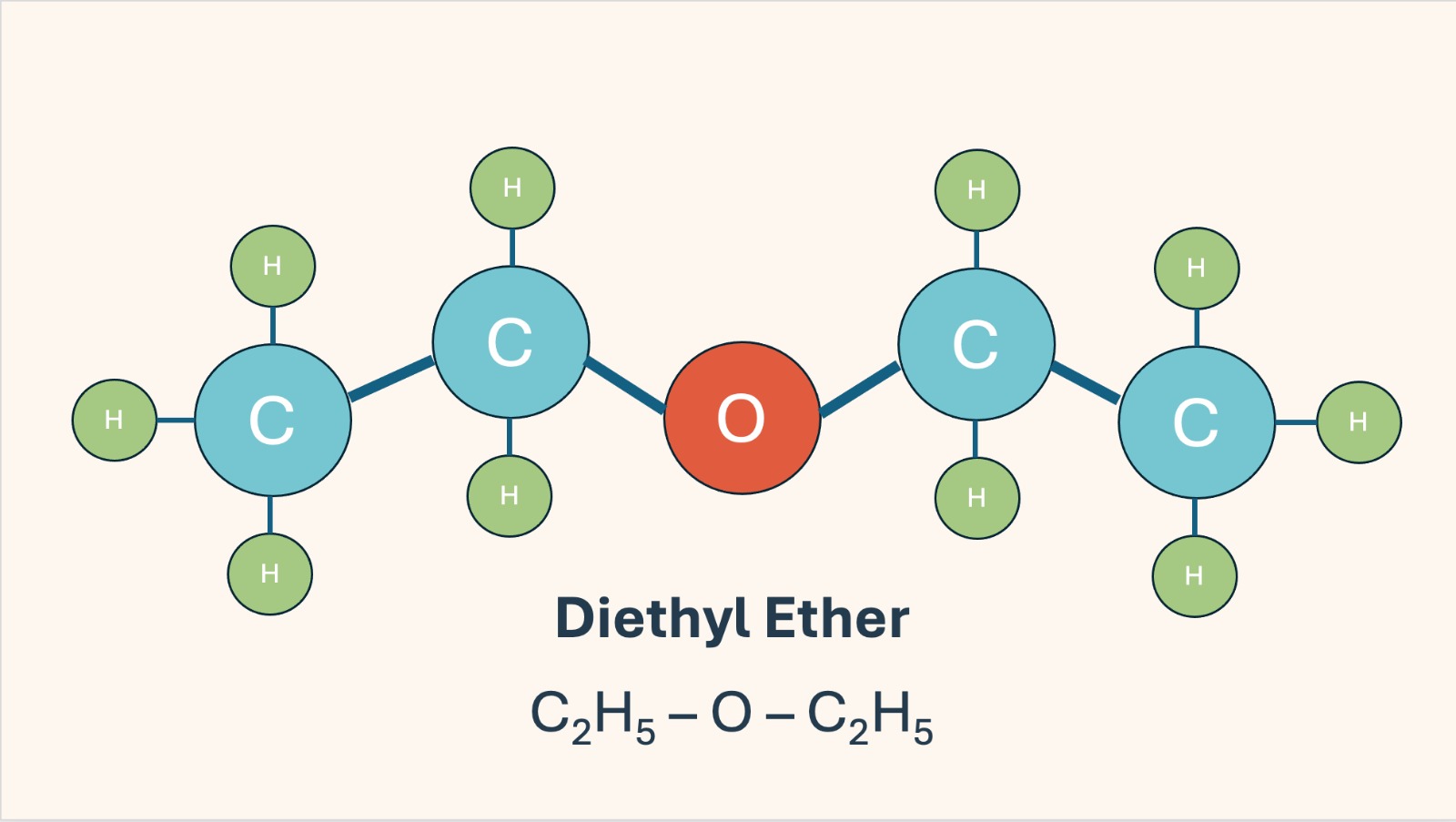
Ether is a clear, highly volatile liquid with a sweet, distinctive smell, a highly flammable organic compound that became the first widely used general anesthetic. It belongs to the ether class of compounds and is characterized by an oxygen atom connected to two ethyl groups.
Chemical formula: C₄H₁₀O or C₂H₅–O–C₂H₅
Structure: Two ethyl groups (C₂H₅) connected by an oxygen atom.
Key properties:
Its rapid absorption and effect on the brain made it ideal for use in anaesthesia. Morton, a Boston dentist, was searching for a better way to perform painless tooth extractions. Ether wasn't unknown—it had been used recreationally at "ether frolics" in the early 1800s, where people would inhale it for fun due to its dizzying effects.

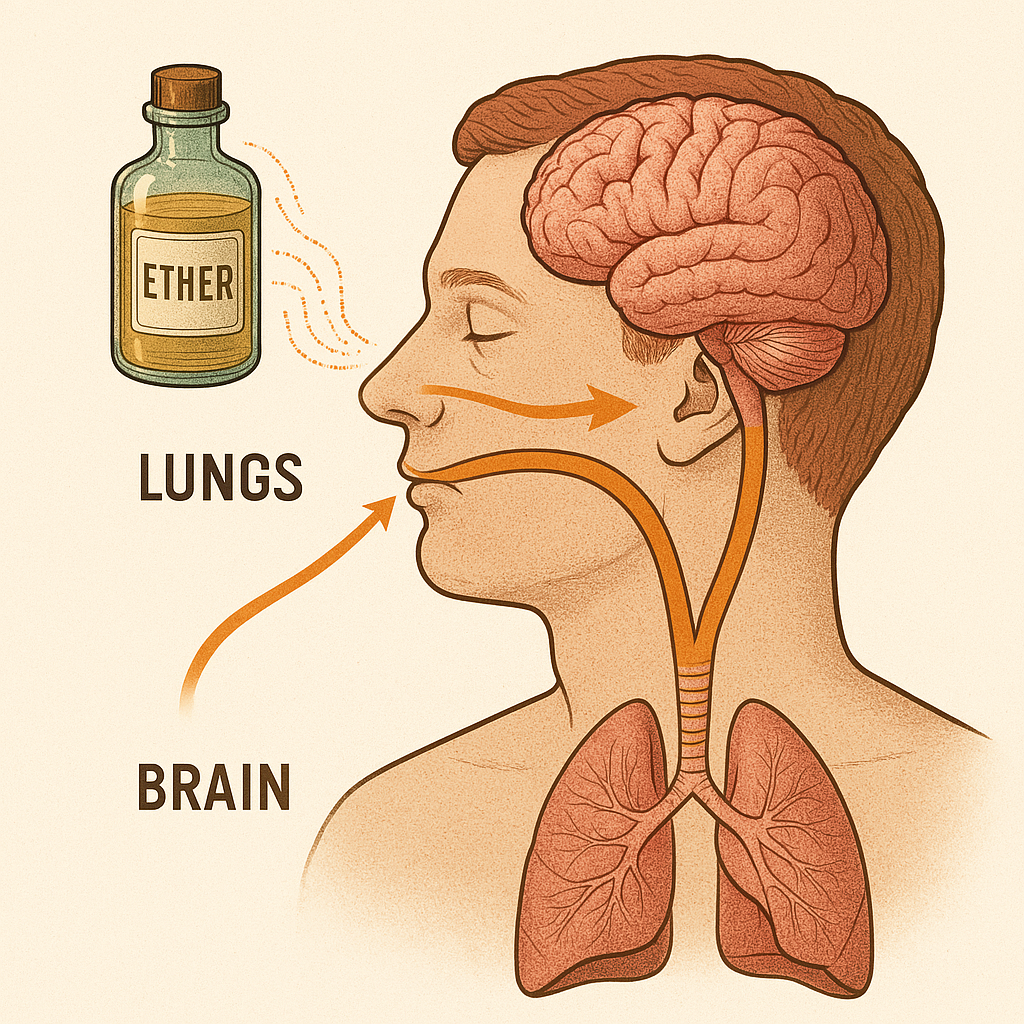


Ether's rapid absorption and effect on the brain made it ideal for use in anaesthesia. Ether wasn't unknown. It had been used recreationally at ether frolics, in the early 1800s, where people would inhale it for fun, due to its dizzying effects.
When diethyl ether entered the surgical scene in the 19th century, it disrupted centuries of brutality in operating rooms. Chemistry unlocked the doorway to sedation, allowing physiology to remain intact while consciousness was suspended. This moment marked a pivotal moment in medical history - when chemistry began to meaningfully transform how pain was understood and managed.
Diethyl ether (C₄H₁₀O) is a small, volatile molecule. When inhaled, it travels quickly from the lungs into the bloodstream, and from there to the brain. This is where chemistry meets physiology.
Once in the brain, ether disrupts the activity of neurons—the nerve cells that process pain and consciousness. It doesn’t block pain like modern painkillers. Instead, it temporarily shuts down the brain’s ability to perceive it. It does this by affecting the lipid membranes and ion channels in nerve cells, silencing their electrical signals. The result? The patient loses consciousness, feels no pain, and doesn’t move.
Ether was powerful, fast-acting, and easy to administer—just a few deep breaths. It let surgeons operate without screams or struggle, changing medicine forever.
This moment marked the beginning of modern surgery—all thanks to a molecule that changed how the brain works, even if just for a short time.